Two mRNA vaccines from Pfizer and Moderna are nearing final approval and both work by rewiring a genetic trigger. The Moderna COVID19 Vaccine does not contain SARSCoV2 or any virusjust the blueprint to help fight against it.
 Moderna Mrna Pfizer Pfe Covid 19 Vaccines Look Strong How They Compare Bloomberg
Moderna Mrna Pfizer Pfe Covid 19 Vaccines Look Strong How They Compare Bloomberg
Stanford scientists have published the mRNA sequence of the Moderna Covid-19 vaccine after reverse-engineering the droplets left in used vials.
Is the moderna vaccine mrna. Once the viral sequences were revealed in January it took just days for pharmaceutical companies Moderna and. Does it work against new variants. It is a medical.
However many years of research have gone into RNA vaccines which is one reason why scientists were able to start testing such vaccines against Covid-19 so quickly. She said that while Moderna and Pfizer are based on new vaccine technologies they are asking our bodies to do something they do every day. SARS-CoV-2 the virus that causes COVID-19 coronavirus has a.
Moderna a Massachusetts-based vaccine developer partnered with the National Institutes of Health to develop and test a coronavirus vaccine known as mRNA-1273. The mRNA Pfizer-BioNTech and Moderna vaccines do not contain live virus and are okay to be given. The monitoring collection and analysis of data on new.
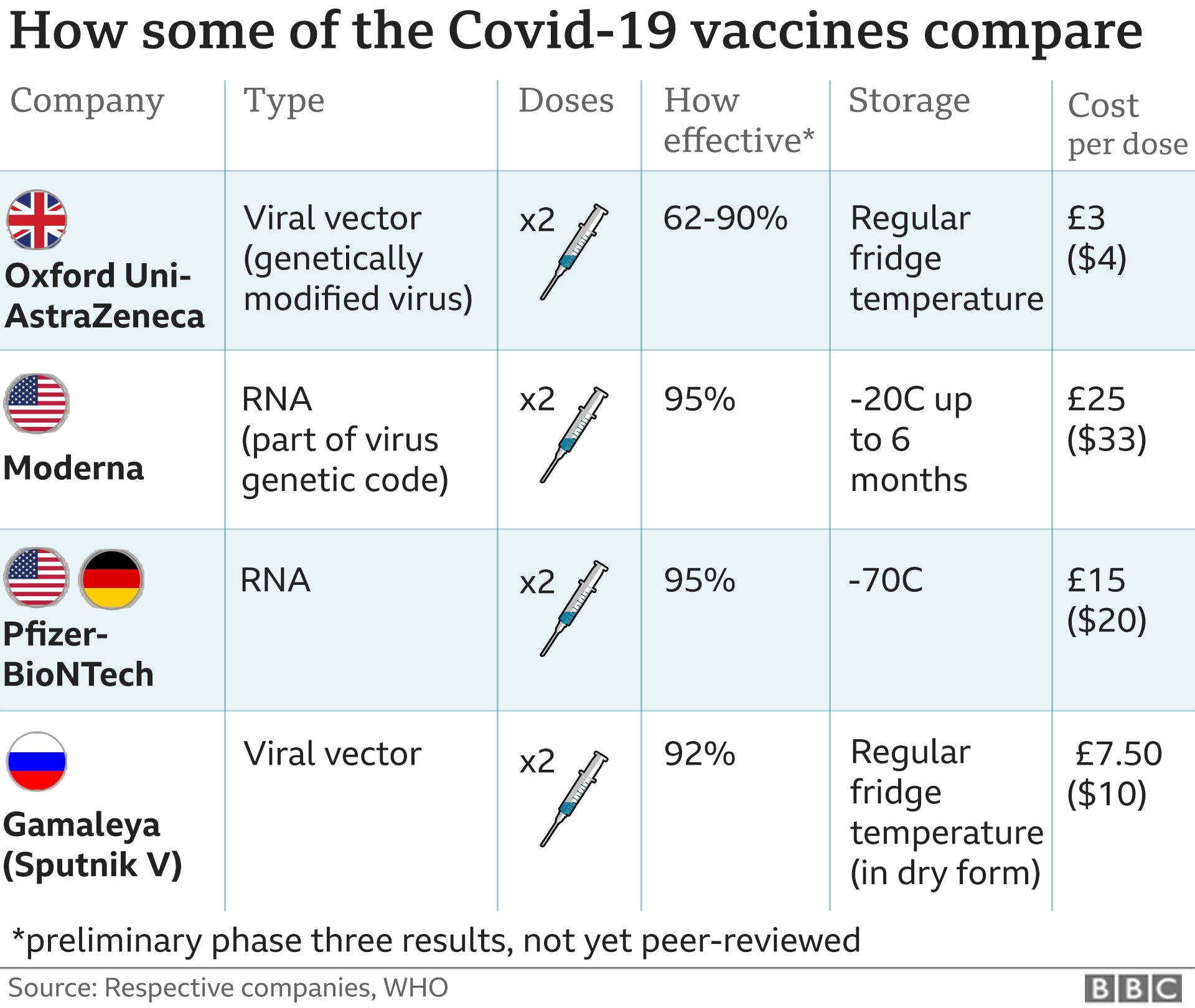
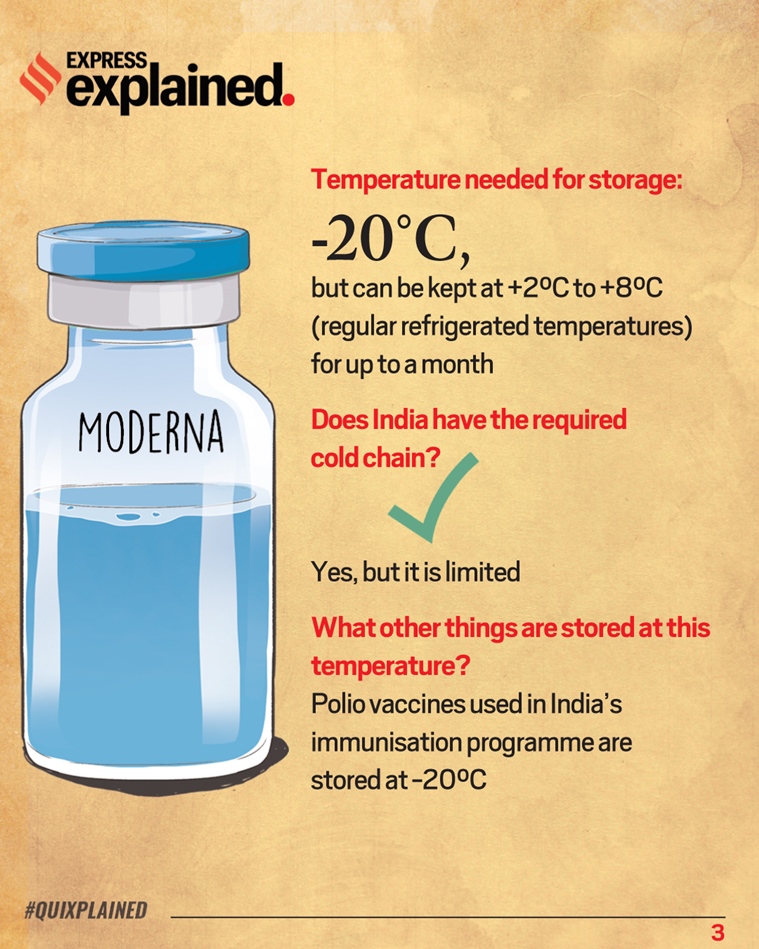
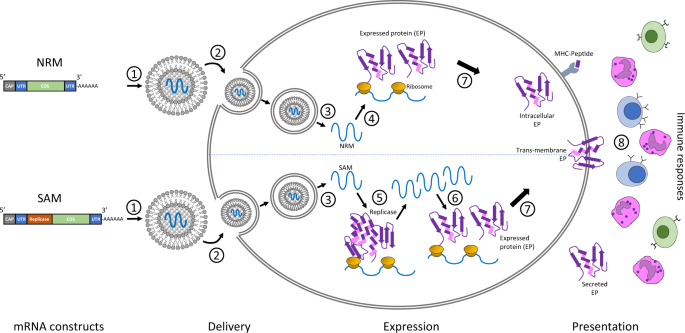
This is a common myth about mRNA vaccines like Modernas as we have written before. To this end both companies have apparently used modified nucleotides to try to make their mRNA molecules more stable The Moderna vaccine in contrast can reportedly be stored at -20C for up to six months which is much more doable as most standard freezers can reach this temperature. This is a mRNA packaged in a fat envelope that is delivered to a cell.
It took researchers just a few days in January 2020 to come up with the mRNA sequence used in Modernas Covid. The powerful technology behind the Pfizer and Moderna vaccines Health Apr 2 2021 644 PM EDT Two of the three COVID-19 vaccines that have. Its the key ingredient in the Pfizer and Moderna COVID-19 vaccines.
David Martin makes some extremely important points about how we cant accurately label the device Moderna and Pfizer are pushing as a vaccine because both medically and legally its not a vaccine. Also mRNAs modular nature makes designing new vaccines relatively straightforward. Using the term vaccine to sneak this thing under public health exemptions.
COVID-19 Vaccine Moderna is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 18 years and older. The Pfizer vaccine and the Moderna vaccine use synthetic mRNA that contains information about the coronaviruss signature spike protein. Based on the evidence so far the new variants of SARS-CoV-2 including the B117 and the 501YV2 do not alter the effectiveness of the Moderna mRNA vaccine.
New Approach to Vaccines. Praising the work of their peers working at Moderna. For hematopoietic stem cell transplant recipients vaccination.
The Moderna COVID19 Vaccine may not protect everyone. To trigger an immune response many vaccines put a weakened or inactivated germ into our bodies. The Moderna vaccine has been shown to have an efficacy of approximately 92 per cent in protecting against COVID-19 starting 14 days after the first dose.
Conventional vaccines usually contain inactivated disease-causing organisms pathogens or proteins made by the pathogen antigens which mimic the infectious agent and cause an immune response in the body which means it can fight the real infection later. MRNA vaccines are a new type of vaccine to protect against infectious diseases. Vaccination should be delayed at least one month from transplant surgery.
2 shots one month 28 days apart. Messenger RNA vaccinesalso called mRNA vaccinesare some of the first COVID-19 vaccines authorized for use in the United States. One surprising star of the coronavirus pandemic response has been the molecule called mRNA.
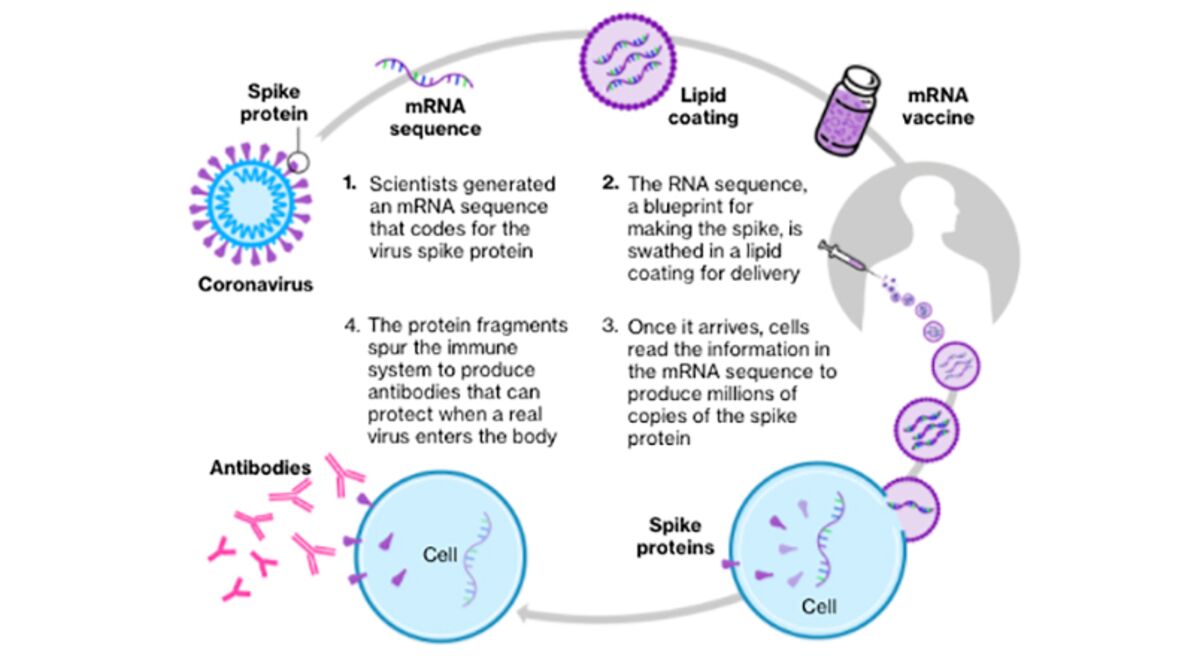
The Moderna COVID19 Vaccine uses mRNA to provide a blueprint for your cells to build your bodys defense against SARSCoV2 the virus that causes COVID19. Vaccination should be offered irrespective of past infection or antibodies For solid organ transplant recipients the ideal timing of vaccination is uncertain. This is not a vaccine.
COVID-19 Vaccine Moderna contains a molecule called messenger RNA mRNA with instructions for producing a protein from SARS-CoV-2 the virus that causes COVID-19. A vaccine based on mRNA has never been approved by the FDA before.